Reaction of HCOOCH CH2 H2O: Guide for Students and Chem Enthusiasts
If you’ve come across the chemical equation involving HCOOH (formic acid), CH2 (methylene group), and H2O (water) + (HCOOCH CH2 H2O) and wondered how they react, you’re not alone. This guide is for chemistry students, enthusiasts, or curious minds who want to understand what happens when these compounds interact.
We’ll explore:
- The nature and behavior of each compound
- The possible reaction pathways
- Real-world applications
- A relatable user story to make things practical
What Are the Components In HCOOCH CH2 H2O?
Let’s begin by understanding each part of the equation.
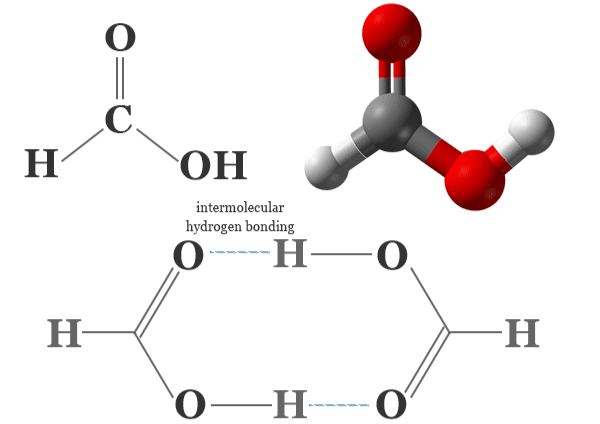
1. HCOOH (Formic Acid)
Formic acid is the handiest carboxylic acid with the chemical formula HCOOH. It occurs naturally in the venom of ant stings and is commonly used in:
- Leather processing
- Preservatives
- Cleaning agents
Properties:
- Weak acid
- Soluble in water
- Reacts with alcohols, amines, and even water under certain conditions
2. CH2 (Methylene Group)
This is a reactive intermediate in organic chemistry. You won’t typically find CH2 floating around freely; it usually appears during reactions or as part of a larger molecule (like CH2Cl2 or CH2=CH2).
Properties:
- Highly reactive
- Often involved in insertion or addition reactions
- Acts as a bridging unit in polymer chains
3. H2O (Water)
Water is more than just a solvent in chemical reactions. In many cases, it acts as a nucleophile, base, or maybe a reactant.
Properties:
- Polar molecule
- Great solvent for ionic and polar substances
- Involved in hydrolysis and acid-base reactions
Possible Reactions Involving HCOOCH CH2 H2O
The combination of formic acid, methylene, and water doesn’t represent a standard textbook reaction. But based on chemistry principles, several reaction scenarios can be considered.
Scenario 1: Hydrolysis in the Presence of Methylene
If methylene acts as a carbene (:CH2), the reaction may follow this pathway:
HCOOH + :CH2 → CH3COOH (Acetic acid or other intermediates depending on conditions)
Here, CH2 inserts into the H-COOH bond, followed by hydrolysis to form a more stable compound.
Scenario 2: Methylene Insertion
Methylene can insert into double bonds or reactive groups. In aqueous solutions, it may facilitate the formation of a longer carbon chain, possibly forming:
- Glycolic acid derivatives
- Hydroxy acids
Scenario 3: Polymerization Potential
In industrial chemistry, methylene bridges are often used in polymer structures (e.g., phenol-formaldehyde resins). In the presence of formic acid and water, controlled environments could initiate polymer chain growth.
A Real-Life Experiment: Student Experience
User Story: Zainab’s Organic Chemistry Lab
Zainab, a second-year undergraduate in Pakistan, conducted an experiment involving methylene chloride, formic acid, and water.
“We were trying to simulate a reaction pathway involving the insertion of CH2 into an acid environment. While we didn’t see the expected yield, we observed a color change and an exothermic effect. Our professor explained that methylene likely initiated a partial hydrolysis process with the formic acid. It wasn’t just theoretical anymore—we saw chemistry in motion.”
This experience reflects how unpredictable but exciting organic chemistry can be, especially with reactive intermediates like CH2.
Practical Applications of These Reactions
Even though CH2 as a free radical is rare, similar reaction pathways are used in:
1. Chemical Manufacturing
- Synthesis of acetic acid derivatives
- Polymer chain formation with formaldehyde or methylene bridges
2. Agriculture
- Formic acid is used in silage preservatives
- Methylene compounds are intermediates in pesticide production
3. Pharmaceuticals
- Designing organic scaffolds with CH2 insertions
- Water plays a vital function in drug solubility and reactivity
Reaction Conditions to Consider
Chemical reactions aren’t just about the ingredients—you also need to control the environment:
| Condition | Importance |
| Temperature | Affects reaction rate and CH2 stability |
| Solvent | Water can act as a medium or reactant |
| Catalyst | May be needed for CH2 formation |
| pH Level | Influences acid-base reactions involving HCOOH |
Common Questions (FAQs)
1. Can CH2 react directly with formic acid?
In theory, yes. As a carbene, CH2 is highly reactive and could insert into the C-H or O-H bonds of formic acid, but it requires controlled lab conditions.
2. Is this a safe reaction to conduct at home?
No. Methylene and formic acid are both reactive and potentially harmful. Always conduct such reactions under expert supervision.
3. What role does water play in this equation?
Water can act as a solvent or as a participant in hydrolysis or hydration reactions, depending on the mechanism.
4. Is this reaction used industrially?
Variants of it are. Formic acid and methylene groups are used in different chemical industries but not typically in this exact trio.
5. What happens if you change the ratio of the components?
Changing the ratio could lead to incomplete reactions or different by-products depending on which component is in excess.
Final Thoughts: Why This Reaction Matters
While the combination of HCOOCH CH2 H2O isn’t a classic go-to reaction, it represents the kind of real-world chemistry that shows up in labs and industry. It teaches us:
- The value of reactive intermediates
- The importance of water in reaction dynamics
- How common compounds can create complex systems
Whether you’re studying for an exam, preparing a research project, or just curious about chemistry, understanding these kinds of reactions enhances your practical knowledge.